# Beyond the Price Tag: How AI Can Define “Clinically Appropriate” in MedTech
The U.K.’s National Institute for Health and Care Excellence (NICE) recently issued a seemingly straightforward recommendation: when performing a transcatheter aortic valve replacement (TAVR), clinicians should use the least expensive, *clinically appropriate* device. While this guidance is rooted in sensible healthcare economics, as an AI practitioner, I see something far more significant. Those two words—”clinically appropriate”—are not a simple qualifier; they represent a complex, multi-dimensional data problem that is a perfect use case for advanced artificial intelligence.
This isn’t just about saving money. It’s about optimizing a high-stakes decision at the individual patient level. The NICE guidance places a new analytical burden on clinical teams, and it’s a burden that AI is uniquely positioned to help carry.
### The Anatomy of a High-Stakes Decision
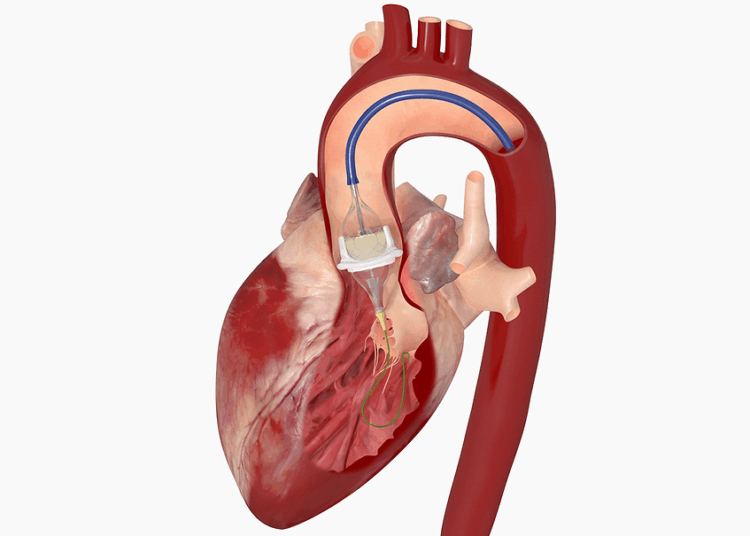
Choosing a TAVR device is not like picking a component off a shelf. It’s a deeply personalized decision that depends on a confluence of factors:
* **Patient Anatomy:** The size and shape of the patient’s aortic annulus, the tortuosity of their femoral arteries, and the extent of calcification are all critical variables, typically assessed through complex imaging like CT scans.
* **Device Specifications:** Each TAVR valve has unique properties—its frame material, expansion mechanism, and leaflet design can influence its performance and suitability for a specific anatomy.
* **Risk Profiles:** The primary goal is a successful valve replacement, but secondary considerations are just as important. What is the risk of a paravalvular leak? What is the likelihood the patient will need a permanent pacemaker post-procedure? What about the long-term durability of the device?
A human expert, the interventional cardiologist, synthesizes this data using their extensive training and experience. But as the number of available devices grows and the pressure to justify cost-effectiveness mounts, this cognitive load becomes immense. How can one definitively prove that Device A, which costs £2,000 more than Device B, is truly the most “clinically appropriate” choice for this specific 82-year-old patient with moderate calcification and a bicuspid valve?
This is where the conversation shifts from clinical intuition to computational intelligence.
### AI as the Decision Support Co-Pilot
The challenge laid out by NICE is an open invitation for AI-driven decision support systems. Imagine a platform that integrates a patient’s entire pre-operative dataset: their electronic health records, high-resolution CT scans, and echocardiogram results. A sophisticated machine learning model, trained on thousands—or even hundreds of thousands—of previous TAVR cases and their long-term outcomes, could then run a simulation for that specific patient.
Instead of a binary “yes/no,” the AI wouldn’t make the decision. It would *illuminate the decision space* for the clinician by providing a dashboard that forecasts outcomes for each available TAVR device:
* **Device A (Lowest Cost):** 98% probability of successful deployment, 12% risk of moderate paravalvular leak, 15% chance of requiring a new pacemaker.
* **Device B (Mid-Cost):** 99% probability of successful deployment, 4% risk of moderate paravalvular leak, 8% chance of requiring a new pacemaker.
* **Device C (Highest Cost):** 99.5% probability of successful deployment, 3% risk of moderate paravalvular leak, 7% chance of requiring a new pacemaker.
Suddenly, the term “clinically appropriate” is no longer a subjective judgment call. It becomes a quantifiable, data-driven conversation between the clinical team and the evidence. The AI provides the probabilistic landscape, and the physician uses their expertise to navigate it. Perhaps for a frail patient, minimizing the risk of a pacemaker is the top priority, making Device B the most “appropriate” choice despite not being the cheapest. For a more robust patient, the risk profile of Device A might be perfectly acceptable.
### Conclusion: From Cost-Cutting Mandate to a Data-Driven Future
NICE’s recommendation, while framed around cost, is a catalyst for innovation. It pushes the medical community beyond simply choosing a device based on brand familiarity or historical preference and toward a system of justifiable, evidence-based selection.
The future of value-based healthcare lies in this synergy between human expertise and machine intelligence. AI’s role is not to replace the clinician but to empower them with predictive insights at a scale no human could ever achieve. By turning vast datasets into personalized, actionable intelligence, we can ensure that choosing the “least expensive, clinically appropriate” option is not a compromise, but a new standard of precision medicine. The TAVR space is just the beginning; this model is the blueprint for nearly every major medical device and treatment decision we will make in the coming decade.
This post is based on the original article at https://www.bioworld.com/articles/723204-nice-tells-docs-to-pay-less-for-tavr-when-possible.